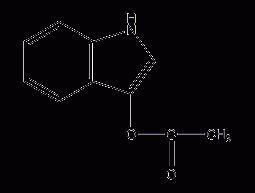
3-indole acetate
Structural formula Business number 06A3 Molecular formula C10H9NO2 Molecular weight 175.18 label indole acetate, Indoxyl hydrochloride, 3-Indoxyl acetate, β-indolizine acetate, Hydroxyindole acetate, Acetic acid oxidizes indole, Indoxyl acetate, Indoxyl acetate Numbering system Physical property data Toxicological data Ecological data Molecular structure data Computing chemical data More Properties and Stability Storage method Synthesis method Purpose Numbering system CAS number:608-08-2 MDL number:MFCD00014561 EINECS number:210-154-2 RTECS number:AI3325000 BRN number:143086…
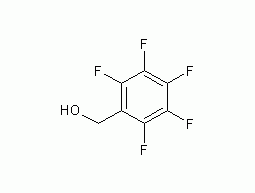
2,3,4,5,6-pentafluorobenzyl alcohol
Structural formula Business number 04U0 Molecular formula C7H3F5O Molecular weight 198 label None yet Numbering system Physical property data Toxicological data Ecological data Molecular structure data Computing chemical data More Properties and Stability Storage method Synthesis method Purpose Numbering system CAS number:440-60-8 MDL number:MFCD00004602 EINECS number:207-126-7 RTECS number:DP0695000 BRN number:2052669 PubChem number:24851790 Physical property data 一 , physical property data Traits :White solid Density (g/mL,25/4℃): Not…
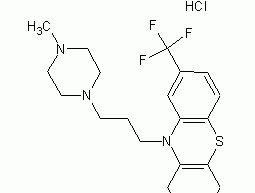
6-Chloro-7-methylpurine
Structural formula Business number 04TZ Molecular formula C21H26Cl2F3N3S Molecular weight 480.42 label 10-[3-(4-Methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-10H-phenothiazine dihydrochloride, Trifluoperazine hydrochloride, strizine, Trifluorazine dihydrochloride, LABOTEST-BB LT00452002, 10-[3-(4-METHYL-1-PIPERAZINYL)PROPYL]-2-TRIFLUOROMETHYL-PHENOTHIAZINE DIHYDROCHLORIDE, 10-[3-(4-METHYLPIPERAZIN-1-YL)PROPYL]-2-(TRIFLUOROMETHYL)-10H-PHENOTHIAZINE DIHYDROCHLORIDE, STELAZINE, STELAZINE DIHYDROCHLORIDE, TRIFLUOPERAZINE 2H Numbering system Physical property data Toxicological data Ecological data Molecular structure data Computing chemical data More Properties and Stability Storage method Synthesis method Purpose Numbering system CAS number:440-17-5 MDL number:MFCD00012656 EINECS number:207-123-0 RTECS number:SP1750000 BRN number:3820024…
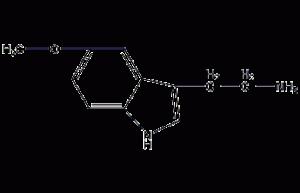
5-methoxytryptamine
Structural formula Business number 06A1 Molecular formula C11H14N2O Molecular weight 190.24 label None yet Numbering system Physical property data Toxicological data Ecological data Molecular structure data Computing chemical data More Properties and Stability Storage method Synthesis method Purpose Numbering system CAS number:608-07-1 MDL number:MFCD00005662 EINECS number:210-153-7 RTECS number:NL4059000 BRN number:145587 PubChem number:24857245 Physical property data 1. Physical property data Melting point :121-123°C Toxicological data None yet…
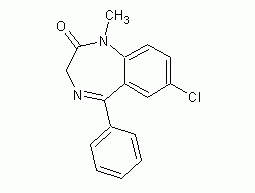
diazepam
Structural formula Business number 04TY Molecular formula C16H13ClN2O Molecular weight 284.74 label 7-Chloro-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2(1H)-one, Ro 5-2807, 7-Chloro-1-methyl-5-phenyl-1,3-dihydro-1,4-benzodiazepine-2-one, 7-Chloro-2,3-dihydro-1methyl-5-phenyl-1,4-benzodiazepin-2, stable, Benzodiazepine, 7-Chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepine-2-one, diazepam, ① Diazepam ② Diazepam (0405 requires application for export license), Benzodiazepine Numbering system Physical property data Toxicological data Ecological data Molecular structure data Computing chemical data More Properties and Stability Storage method Synthesis method Purpose Numbering system CAS number:439-14-5 MDL number:MFCD00057323 EINECS number:207-122-5 RTECS number:DF1575000…
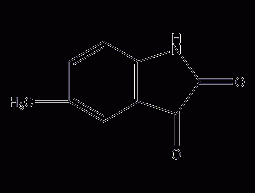
5-methylisatin
Structural formula Business number 06A0 Molecular formula C9H7NO2 Molecular weight 161.16 label 5-methyl-2,3-indoledione, 5-methylindole-2,3-dione, 5-Methylindole-2,3-dione Numbering system Physical property data Toxicological data Ecological data Molecular structure data Computing chemical data More Properties and Stability Storage method Synthesis method Purpose Numbering system CAS number:608-05-9 MDL number:MFCD00005721 EINECS number:210-152-1 RTECS number:NL7939300 BRN number:123738 PubChem number:24896609 Physical property data 1. Characteristics: purple-red Crystal 2. Melting point:184-188℃ Toxicological data None…
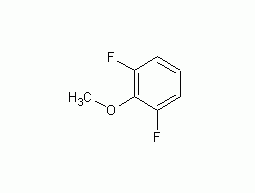
2,6-Difluoroanisole
Structural formula Business number 04TX Molecular formula C7H6F2O Molecular weight 144.12 label 2,6-Difluoroanisole, 2,6-Difluoroanisole, 97+%, 2,6-DIFLUOROANISOLE, 2,6-Difluoroanisole, 97+%, 2,6-Difluoroanisole 98%, 2,6-Difluoroanisole98%, 2,6-DIFLUOROANISOLE: 98.5% Numbering system Physical property data Toxicological data Ecological data Molecular structure data Computing chemical data More Properties and Stability Storage method Synthesis method Purpose Numbering system CAS number:437-82-1 MDL number:MFCD00142846 EINECS number:None RTECS number:None BRN number:None PubChem ID:None Physical property data 一 ,…
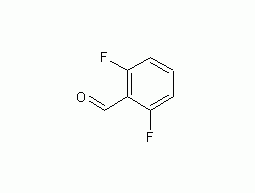
2,6-Difluorobenzaldehyde
Structural formula Business number 04TW Molecular formula C7H4F2O Molecular weight 142.10 label 2,6-Difluorobenzaldehyde, 2,6-Difluorobenzaldehyde, 97%, 3,4,5-Trifluorobenzonitrile, 2,6-DIFLUOROBENZALDEHYDE, TIMTEC-BB SBB006685, 2,6-Difluorobenzaldehyde,97%, 3,4,5-triflurobenzenenitrile, 2,6-Difluorobenzaldehyde 98%, 2,6-Difluorobenzaldehyde98%, 2,6-DIFLUORLOBENZALDEHYDE, 2-6-Difluorbenzaldehyd Numbering system Physical property data Toxicological data Ecological data Molecular structure data Computing chemical data More Properties and Stability Storage method Synthesis method Purpose Numbering system CAS number:437-81-0 MDL number:MFCD00010293 EINECS number:000-000-0 RTECS number:None BRN number:1935273 PubChem number:24856057 Physical property…
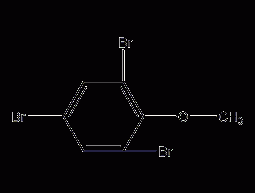
2,4,6-Tribromoanisole
Structural formula Business number 069Z Molecular formula C7H5Br3O Molecular weight 344.83 label 2,4,6-Tribromo-1-methoxybenzene, 2,4,6-Tribromo-1-methoxybenzene, Br3C6H2OCH3 Numbering system Physical property data Toxicological data Ecological data Molecular structure data Computing chemical data More Properties and Stability Storage method Synthesis method Purpose Numbering system CAS number:607-99-8 MDL number:MFCD00192510 EINECS number:None RTECS number:None BRN number:2210361 PubChem number:24864766 Physical property data 1. Physical property data Boiling point: 297℃Melting point: 87-89℃ Toxicological…
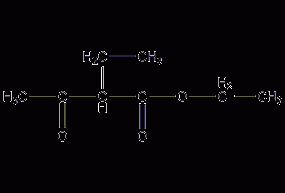
2-Ethyl acetoacetate
Structural formula Business number 069Y Molecular formula C8H14O3 Molecular weight 158.19 label Ethyl α-ethylbutanate, 2-Acetylbutyrate ethyl ester, CH3COCH(C2H5)COOC2H5 Numbering system Physical property data Toxicological data Ecological data Molecular structure data Computing chemical data More Properties and Stability Storage method Synthesis method Purpose Numbering system CAS number:607-97-6 MDL number:MFCD00039898 EINECS number:210-151-6 RTECS number:None BRN number:636286 PubChem number:24845390 Physical property data Physical property data: 1. Density 0.981 2.…



