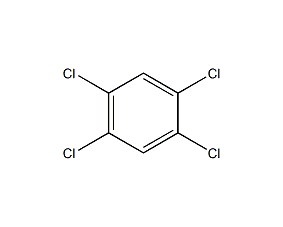
Structural formula
| Business number | 02A7 |
|---|---|
| Molecular formula | C6H2Cl4 |
| Molecular weight | 215 |
| label |
None |
Numbering system
CAS number:95-94-3
MDL number:MFCD00000549
EINECS number:202-466-2
RTECS number:DB9450000
BRN number:1618315
PubChem number:24848038
Physical property data
1. Character: white flakes[1]
2. Melting point (℃): 139~142[2]
3. Boiling point (℃): 243~246[3]
4. Relative density (water=1): 1.73 (10℃)[4 ]
5. Relative vapor density (air=1): 7.4[5]
6. Saturated vapor pressure (kPa): <0.013 (25℃)[6]
7. Critical temperature (℃): 489.8[7]
8 .Critical pressure (MPa): 3.38[8]
9. Octanol/water partition coefficient: 4.64[9]
10. Flash point (℃): 155 (CC) [10]
11. Solubility: insoluble in water, slightly soluble in ethanol, soluble in benzene and ether and chloroform. [11]
12. Vapor pressure temperature (ºC, 5.33kPa): 146
13. Vapor pressure temperature (ºC, 8.0kPa) :157.7
14. Vapor pressure temperature (ºC, 13.3kPa): 173.5
15. Vapor pressure temperature (ºC, 26.7kPa): 196
16. Vapor pressure temperature (ºC, 53.3kPa): 220.3
17. Vapor pressure temperature (ºC, 101.3kPa): 245
18. Gas phase standard entropy (J ·mol-1·K-1): 393.60
19. Gas phase standard hot melt (J·mol-1·K-1):144.79
Toxicological data
1. Acute toxicity[20] LD50: 1500mg/kg (rat oral)
2. Irritation No data yet
3. Subacute and chronic toxicity [21] Rabbit inhalation contains 20% tetrachlorobenzene (concentration 4~ 5g/m3 or 8~10g/m3) powder for 1 to 17 days, resulting in a decrease in red blood cells and hemoglobin and an increase in lymphocytes. When rats were fed 0.005 mg/kg, the conditioned reflex activity was changed, the sulfhydryl content in the serum was reduced, and the liver function was slightly disordered.
Ecological data
1. Ecotoxicity[22]
LC50: 4.35mg/L (48h), 1.55mg/L (96h) (blue gill sunfish); 26.4mg/L (48h) (medaka)
2. Biodegradability[23]
Aerobic biodegradation (h): 672~4320
Anaerobic biodegradation (h): 2880~17280
3. Non-biodegradability [24]
Photooxidation half-life in air (h): 763.1~7631
First-order hydrolysis half-life (h): >879a
4. Bioaccumulation [25]
BCF: 2720~4830 (carp, contact concentration 10ppb, contact time 8 weeks ); 1650~3930 (carp, exposure concentration 1ppb, exposure time 8 weeks)
Molecular structure data
1. Molar refractive index: 45.83
2. Molar volume (cm3/mol): 137.2
3. Isotonic specific volume (90.2K ): 350.7
4. Surface tension (dyne/cm): 42.6
5. Dielectric constant:
6. Dipole moment (10 -24cm3):
7. Polarizability: 18.16
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 90.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[26] Stable
2. Incompatible substances[27] Strong oxidants, strong alkalis
3. Conditions to avoid contact[28] Heating
4. Polymerization hazard[29] No polymerization
5. Decomposition products[30] Hydrogen chloride
Storage method
Storage Precautions[31] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Prepared from chlorination of o-dichlorobenzene.
Purpose
Used as an intermediate in organic synthesis. [32]



