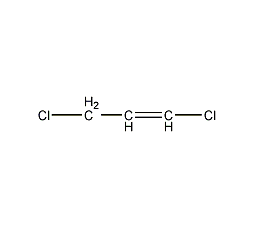
Structural formula
| Business number | 05LQ |
|---|---|
| Molecular formula | C3H4Cl2 |
| Molecular weight | 110 |
| label |
drip mixture, drip mixture, drops, 1,3-Dichloro-1-propene, 2-Chloropropenyl chloride, o-dichloropropylene, 1,3-Dichloropropene, Trans-1,3-Dichloro-1-Propene, Trans-1,3-Dichloropropylene |
Numbering system
CAS number:542-75-6
MDL number:MFCD00000985
EINECS number:208-826-5
RTECS number:UC8310000
BRN number:1719556
PubChem ID:None
Physical property data
1. Properties: Amber liquid with a chloroform-like smell. [1]
2. Melting point (℃): -84[2]
3. Boiling point (℃): 108[3]
4. Relative density (water = 1): 1.22[4]
5. Relative vapor Density (air=1): 3.8[5]
6. Saturated vapor pressure (kPa): 4.5 (25℃)[6]
7. Heat of combustion (kJ/mol): -1775.5[7]
8. Octanol/water partition coefficient: 1.82[8 ]
9. Flash point (℃): 25 (CC); 35 (OC) [9]
10. Explosion limit (%): 14.5[10]
11. Lower explosion limit (%): 5.0[11]
12. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol, ether, and benzene. [12]
Toxicological data
1. Acute toxicity[13]
LD50: 470~710mg/kg (rat oral ); 640mg/kg (oral in mice); 775mg/kg (transdermal in rats); 504mg/kg (transdermal in rabbits)
LC50: 500ppm (rat inhalation); 4650mg/m3(mouse inhalation, 2h)
2. Irritation No data available
3. Subacute and Chronic toxicity[14] Rats inhaled 50ppm, 6 hours a day, for 12 weeks, the liver and kidneys were enlarged, but the animals survived.
4. Mutagenicity[15] Microbial mutagenicity: Salmonella typhimurium 100μg/dish. Sister chromatid exchange: hamster ovary 900nmol/L. DNA damage: mice were given 150 mg/kg intraperitoneally.
5. Carcinogenicity[16] IARC Carcinogenicity Comment: G2B, suspected human carcinogen.
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[17]
Aerobic biodegradation (h): 168~672
Anaerobic biodegradation (h): 672~2688
3 .Non-biodegradability[18]
Photooxidation half-life in air (h): 4.66~80.3
First-order hydrolysis half-life (h): 271
Molecular structure data
1. Molar refractive index: 25.70
2. Molar volume (cm3/mol): 94.4
3. Isotonic specific volume (90.2K ): 215.6
4. Surface tension (dyne/cm): 27.1
5. Polarizability (10-24cm3):10.18
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 31.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[19] Stable
2. Incompatible substances[20] Strong oxidants, acids
3. Conditions to avoid contact[21] Heating
4. Polymerization hazards[22] Polymerization
5. Decomposition products[23] Hydrogen chloride, light Qi
Storage method
Storage Precautions[24] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants and acids, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
It can be separated from the by-product of propylene chloride produced from high-temperature chlorination of propylene. It can also be obtained by dehydrochlorination of 1,2,3-trichloropropane under the action of alkali.
Purpose
1. It can be mixed with dichloropropane as a soil fumigant, and can also be used as a chemical reagent and solvent to treat the soil before crop planting. It can prevent and control root-knot nematodes, short-body nematodes, cyst nematodes and other nematodes in various crops. and underground pests.
2. Used in organic synthesis and as an antifungal agent. [25]



