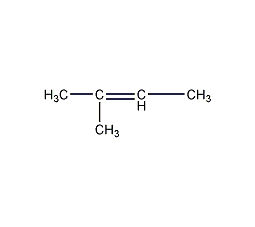
Structural formula
| Business number | 05AM |
|---|---|
| Molecular formula | C5H10 |
| Molecular weight | 70.13 |
| label |
Trimethylethylene, β-Isoamylene |
Numbering system
CAS number:513-35-9
MDL number:MFCD00009276
EINECS number:208-156-3
RTECS number:EM7650000
BRN number:1361353
PubChem number:24896827
Physical property data
1. Properties: Colorless and volatile liquid with unpleasant odor. [1]
2. Melting point (℃): -133.61[2]
3. Boiling point (℃): 35~38[3]
4. Relative density (water=1): 0.66[4]
5. Relative vapor density (air = 1): 2.4[5]
6. Saturated vapor pressure (kPa): 96.3 (37.8℃)[6]
7. Critical temperature (℃): 197.8[7]
8. Critical pressure (MPa): 3.44[8]
9. Octanol/water partition coefficient: 2.67[9]
10. Flash point (℃): -45.56 [10]
11. Ignition temperature (℃): 365[11]
12. Explosion limit (%): 8.7 [12]
13. Lower explosion limit (%): 1.6[13]
14. Solubility: insoluble in Water, soluble in most organic solvents such as ethanol and ether. [14]
15. Solubility parameter (J·cm-3)0.5:15.247
16. Eccentricity factor: 0.277
17. van der Waals area (cm2·mol-1): 8.050×109
18. van der Waals volume (cm3·mol-1): 54.5490
19. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -3328.62
20. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -68.07
21. Liquid phase standard entropy (J·mol-1·K-1): 251.2
22. Liquid phase standard free energy of formation (kJ·mol-1): 60.24
23. Liquid phase standard hot melt (J·mol -1·K-1): 152.80
24. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3355.69
25. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -41.00
26. Gas phase standard Entropy (J·mol-1·K-1): 338.7
27. Gas phase standard formation free energy (kJ·mol– 1): 61.4
28. Gas phase standard hot melt (J·mol-1·K-1): 105.0 p>
Toxicological data
1. Acute toxicity No data available
2. Irritation No data available
Ecological data
1. This substance may be harmful to the environment, and special attention should be paid to water bodies.
2. Ecotoxicity No data available
3. Biodegradability No data
4. Non-biodegradability[15] In the air, when the hydroxyl radical concentration is 5.00×105 pcs/cm3, the degradation half-life is 4.4h (theoretical).
Molecular structure data
1. MooreEmissivity: 25.11
2. Molar volume (cm3/mol): 104.4
3. Isotonic specific volume (90.2K): 214.4
4. Surface tension (dyne/cm): 17.7
5. Dielectric constant (F/m): 2.54
6. Polarizability (10-24cm3): 9.95
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 38
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[16] Stable
2. Incompatible substances [17] Oxidants, acids, halogenated hydrocarbons, halogens, etc.
3. Conditions to avoid contact[18] Heating
4. Polymerization hazard[19] Aggregation
Storage method
Storage Precautions[20] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 29°C. The packaging must be sealed and must not come into contact with air. should be kept away from oxidizer, do not store together. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. In the C5 fraction of catalytically cracked gasoline in a refinery, isopentene (including three isomers) accounts for about 34%. In the C5 fraction of the by-product of cracking ethylene in a petrochemical plant, isopentene Accounting for about 9%. When isoprene is extracted from the C5 fraction using a solvent method, isoprene can be separated and produced.
2. Preparation method:
![]()
In a reaction bottle equipped with a fractionation device, add 65 mL of water and 34 mL of concentrated sulfuric acid. After cooling slightly, add 44 g (0.5 mol) of 2-methyl-2-butanol (2), and then add a few grains of zeolite. . Cool the receiving bottle with ice water. Heat the reaction flask in a water bath, and the product will continue to steam out. Adjust the heating so that the temperature at the top of the fractionation column does not exceed 43°C. The distillation rate is controlled at 1 drop/second. The reaction was stopped when the temperature exceeded 42°C. The distillate was washed with 10 mL (10%) of sodium hydroxide and water, and dried over anhydrous calcium phosphate to obtain 21 g of 2-methyl-2-butene (1) with a yield of 70%. [22]
3. Preparation method:
![]()
In a reaction flask equipped with a stirrer and a 20cm long fractionating column (connected to a distillation device and cooled by an ice bath in the bottle), add 2-methyl-2 -25g (0.28mol) butanol (2), 10mL 85% phosphoric acid, mix evenly. Add zeolite, heat slowly, collect fractions at 35-38°C, and complete the reaction in about 30 minutes. The distillate was dried with 1 to 2 g of anhydrous magnesium sulfate, distilled, and the fraction at 37 to 38°C was collected to obtain 12.5 g of product (2) with a yield of 64%. [23]
Purpose
1. Used for dehydrogenation to produce isoprene, and also used as an intermediate for synthetic rubber, resin and organic synthesis. A blending agent that increases the octane rating of gasoline. Gas chromatography comparison sample.
2. Used in organic synthesis. [21]



