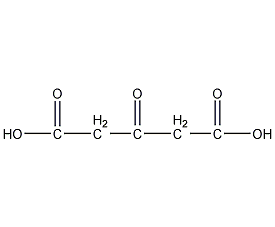
Structural formula
| Business number | 05L9 |
|---|---|
| Molecular formula | C5H6O5 |
| Molecular weight | 146.10 |
| label |
1,3-acetonedicarboxylic acid, 3-pentanonedioic acid, 3-oxoglutaric acid, Beta-glutinic acid, Beta-ketoglutarate, Acetone diacetic acid, Acetonicarboxylic acid, 1,3-Acetonedicarboxylic acid, 3-Oxoglutaric acid |
Numbering system
CAS number:542-05-2
MDL number:MFCD00002711
EINECS number:208-797-9
RTECS number:None
BRN number:1447081
PubChem ID:None
Physical property data
1. Properties: Colorless needle-like crystals
2. Density (g/ cm3, 25/4℃): Undetermined
3. Relative vapor density (g/cm3, air=1): Undetermined
4. Melting point (ºC): 133
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 66.70kPa): Undetermined
7. Refractive index: Undetermined
8 . Flash point (ºF): Not determined
9. Specific rotation (º): Not determined
10. Autoignition point or ignition temperature (ºC): Not determined
p>
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 55.1ºC): Undetermined
13. Heat of combustion ( KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. The logarithmic value of the oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water and alcohol, insoluble in chloroform and benzene, slightly soluble in ether and ethyl acetate
Toxicological data
None
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 28.37
2. Molar volume (cm3/mol): 97.4
3. Isotonic specific volume (90.2K ): 279.7
4. Surface tension (dyne/cm): 67.9
5. Polarizability (10-24cm3):11.24
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.7
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: 5
6. Topological molecule polar surface area 91.7
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 153
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Use and store according to specifications, no decomposition will occur, and avoid contact with oxides
Storage method
Stored at 2-8°C in a cool, dry, well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. It is prepared by using citric acid as raw material and removing formic acid in the presence of concentrated sulfuric acid. In the industrial process, fuming sulfuric acid is first put into the reaction tank, and when it is cooled to below 50°C, citric acid is added in portions with stirring. The weight ratio of ingredients is citric acid: oleum 1:2.34. After adding, raise the temperature to 40-45°C, keep the reaction for 3 hours, then lower the temperature to below 10°C, add normal water dropwise (keep the temperature below 40°C), and then cool to about 15°C, centrifuge, rinse with ice water, and spin dry to obtain the finished product.
2. Preparation method:
Into a 3L reaction bottle ① equipped with a stirrer and thermometer, add 1500g (780mL) of oleum 20% ), cool to -5°C in an ice-salt bath, slowly add 450g (2.14mol) of finely ground citric acid monohydrate (2) ② in batches while stirring, and control the temperature not to exceed 0°C, about Completed in 3 hours. Slowly rise to room temperature, and a large number of bubbles (carbon monoxide gas) will be generated. Stir the reaction until the liquid turns into a light yellow clear liquid and no carbon monoxide emerges. Cool to about 0℃, slowly pour into 800g of crushed ice while stirring, and a white solid will precipitate. Filter with suction, wash with cold water, then wash with ethyl acetate ③, and dry to obtain acetone dicarboxylic acid (1) 266g, yield 85%, mp 132~134℃ (decomposition), sealed and stored at low temperature . Note: ① The reaction vessel should be larger because carbon monoxide is generated during the reaction and a large amount of foam is produced. This should be done under well-ventilated conditions. ②Citric acid should be ground finely, otherwise the lumps will not easily participate in the reaction due to the viscosity of the reaction. ③The product has poor stability and is easy to hydrolyze. If the purity is not high, it will decompose faster. Please pay attention to low temperature storage or use it as soon as possible. [1]
Purpose
Pharmaceutical intermediates, used for anticholinergic drugs such as atropine and anisodamine.



