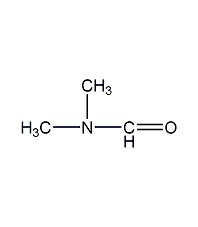
Structural formula
| Business number | 01F6 |
|---|---|
| Molecular formula | C3H7NO |
| Molecular weight | 73 |
| label |
dimethylformamide, formamide, dimethylformamide, N-formamidedimethylamide;, Dimethylformamide, Dormyl dimethylamine, DMF, DMFA, N-Formyldimethylamine, Aliphatic nitrogenous compounds |
Numbering system
CAS number:68-12-2
MDL number:MFCD00003284
EINECS number:200-679-5
RTECS number:LQ2100000
BRN number:605365
PubChem number:24893883
Physical property data
1. Properties: colorless, transparent or light yellow liquid with fishy smell. [1]
2. Melting point (℃): -61[2]
3. Boiling point (℃): 153[3]
4. Relative density (water = 1): 0.95[4]
5. Relative vapor Density (air=1): 2.51[5]
6. Saturated vapor pressure (kPa): 0.5 (25℃)[6]
7. Heat of combustion (kJ/mol): -1921[7]
8. Critical temperature (℃): 374[8]
9. Critical pressure (MPa): 4.48[9]
10. Octanol/water partition coefficient: -0.87[10]
11. Flash point (℃): 58 (OC) [11]
12. Ignition temperature ( ℃): 445[12]
13. Explosion upper limit (%): 15.2[13]
14. Explosion Lower limit (%): 2.2[14]
15. Solubility: miscible with water and miscible in most organic solvents. [15]
16. Refractive index (25ºC): 1.42817
17. Viscosity (mPa·s, 25ºC): 0.802
18. Specific rotation (º): 0.94
19. Flash point (ºC): 445
20. Heat of evaporation (KJ/mol, 25ºC): 47.545
21. Heat of evaporation (KJ/mol, 100ºC): 43.585
22. Heat of evaporation (KJ/mol, b.p.): 38.368
23. Heat of fusion (KJ /mol): 16.165
24. Heat of combustion (KJ/mol): 1915.46
25. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 2.14
26. Electrical conductivity (S/m): 6×10-8
27. Thermal conductivity (W/(m·K), 20ºC ): 0.16579
Toxicological data
1. Acute toxicity[16]
LD50: 4000mg/kg (rat oral); 4720mg/kg (rabbit dermal )
LC50: 9400mg/m3 (mouse inhalation, 2h)
2. Irritation [17] sup> Rabbit eye: 100%, severe irritation (rinse with water)
3. Subacute and chronic toxicity [18] When rats inhaled 2500mg/m3, 6 hours a day for 5 days, 8 to 10 out of 16 died. Liver and lung damage could be seen at autopsy.
Ecological dataDistillation to obtain finished products.

2. Dimethylamine-carbon monoxide Method: Obtained from the direct reaction of dimethylamine and carbon monoxide under the action of sodium methoxide. Reaction conditions are 1.5-2.5MPa and 110-150°C. The crude product is distilled to obtain the finished product.
![]()
3. Made of carbon monoxide and methanol in Methyl formate is obtained through oxo synthesis under high pressure and temperature of 80-100°C, and then reacts with dimethylamine to form dimethylformamide, and the finished product is obtained after distillation.
![]()
4. Trichloroacetaldehyde method : Obtained from the reaction of trichloroacetaldehyde and dimethylamine.
Add chloroform and 0.52 parts of trichloroacetaldehyde into the reaction kettle, cool it to below 30°C, introduce gaseous dimethylamine, and at the same time, add 0.78 parts of trichloroacetaldehyde dropwise into the reaction kettle. , react. After the reaction, a distillation operation is performed. When the temperature at the top of the distillation tower is 58-64°C, the fraction is chloroform, and the fraction at 64-150°C is a mixture of chloroform and dimethylformamide. This mixture is distilled under reduced pressure. The crude dimethylformamide is obtained, and then the crude crystal is distilled to obtain the finished product.
![]() Consumption quota (kg/t): dimethylamine (40%) 2372; Trichloroacetaldehyde (95%) 2543.
Consumption quota (kg/t): dimethylamine (40%) 2372; Trichloroacetaldehyde (95%) 2543.
Refining method: N,N-dimethylformamide often contains impurities such as water, ethanol, primary amines, and secondary amines, and can form HCON(CH3)2·2H2O with 2 molecules of water. To obtain high-purity products, a combination of desiccant and distillation can be used. First, add 1/10 volume of benzene and perform azeotropic distillation under normal pressure to remove water. Then refine according to the following method:
① Add anhydrous magnesium sulfate (25g/L) to dry, and distill under reduced pressure of 2~2.67KPa.
② Add powdered barium oxide, stir, pour out the liquid, and distill under reduced pressure.
③ Add alumina powder (50g/L, calcined at 500~600℃), mix and shake, and distill under reduced pressure (0.67~1.33KPa).
④ Add triphenylsilyl chloride (5~10g/L), heat at 120~140℃ for 24 hours and then distill under reduced pressure (0.67KPa).
The conductivity of the product obtained by the above method: (1) (0.9~1.5)×10-7 S/m; (2) (0.4~1.0)×10-7 S/m; (3) (0.3~0.9)×10-7 S/m; (4) (0.2~0.5)×10-7 S/m.
5. Use industrial product dimethylformamide as raw material and purify to obtain reagent dimethylformamide. If industrial products contain a small amount of water, it can be removed through 4A molecular sieves. If the moisture content is high, you can add an appropriate amount of granular potassium hydroxide, and leave it to stratify without shaking. After removing the water layer containing impurities such as formic acid, add reagent-grade benzene with 1/5 of the volume of dimethylformamide. Distillation under normal pressure. When the gas phase temperature reaches 130°C, add an appropriate amount of phosphorus pentoxide to the residual liquid, cover it and shake for 3.5 hours. After letting it stand, filter out the solid, then dehydrate it with potassium hydroxide under nitrogen-filled conditions, and then Distill under reduced pressure under the protection of dry nitrogen, and collect the middle fraction to obtain a high-purity product.
6. Dimethylamine and carbon dioxide are synthesized under pressure under the catalysis of sodium methoxide or dimethylamine and methyl formate are reacted in the gas phase. It can also be obtained by the reaction of dimethylamine and trichloroacetaldehyde.
Purpose
1. Dimethylformamide is a good solvent for a variety of polymers such as polyethylene, polyvinyl chloride, polyacrylonitrile, polyamide, etc., and can be used for wet spinning of synthetic fibers such as polyacrylonitrile fiber. , Synthesis of polyurethane; used in plastic film making; can also be used as a paint remover for removing paint; it can also dissolve some low-solubility pigments, giving the pigments the characteristics of dyes. Dimethylformamide is used for the extraction of aromatic hydrocarbons and for the separation and recovery of butadiene from the C4 fraction and the separation and recovery of isoprene from the C5 fraction. It can also be used as an effective reagent for the separation of non-hydrocarbon components from paraffin. .
2. It has good selectivity for the solubility of isophthalic acid and terephthalic acid: the solubility of isophthalic acid in dimethylformamide is greater than that of terephthalic acid in dimethylformamide. The two can be separated by solvent extraction or partial crystallization from formic acid formamide. In the petrochemical industry, dimethylformamide can be used as a gas absorbent to separate and refine gases.
3. In organic reactions, dimethylformamide is not only widely used as a solvent for reactions, but also an important intermediate in organic synthesis. It can be used in the pesticide industry to produce pyrimidine.
4. Reagents for titration of non-aqueous solutions, solvents for vinyl resin and acetylene, organic synthesis, photometry, gas chromatography stationary solution (maximum operating temperature 50°C, solvent is methanol), separation and analysis of C2-C5 hydrocarbons , and can separate normal and isobutylene] and cis and trans-butylene. Pesticide residue analysis. Organic Synthesis. Synthesis of peptides. For use in the photographic industry.
5. An excellent solvent and chemical raw material with extremely wide uses. It is an excellent solvent for a variety of polymers such as polyethylene, polyvinyl chloride, polyacrylonitrile, polyamide, etc. It can be used for wet spinning of synthetic fibers such as polyacrylonitrile fiber; synthesis of polyurethane; paint remover for paint removal; selective absorption of acetylene and separation and refining of butadiene; used as a solvent in the production of artificial leather; used in pesticides Used to synthesize pesticides; in the pharmaceutical industry, it can be used to synthesize iodine, doxycycline, cortisone, vitamin B6, iodine glycosides, pyrantel, pyrantel, N-formylsarcolysin, and antineoplasmic acid. , methoxychloride, benzene mustard, cyclohexyl nitrosourea, furfurouracil, hemostatic acid, befenmethane, megestrol, cholevitamin, chlorpheniramine, etc.
6. Used as a solvent and organic modifier in liquid chromatography, an extractant and developing agent for thin layer chromatography analysis, a solvent for vinyl resin and acetylene, and a solvent for titration of non-aqueous solvents. and used in organic synthesis.
7. Mainly used as industrial solvents, in the pharmaceutical industry for the production of hormones, and also for the manufacture of pyroxime. [25]
Hexyl nitrosourea, furfururacil, tournexamic acid, sefenmethasone, megestrol, cholevitamin, chlorpheniramine, etc.
6. Used as a solvent and organic modifier in liquid chromatography, an extractant and developing agent for thin layer chromatography analysis, a solvent for vinyl resin and acetylene, and a solvent for titration of non-aqueous solvents. and used in organic synthesis.
7. Mainly used as industrial solvents, in the pharmaceutical industry for the production of hormones, and also for the manufacture of pyroxime. [25]



